生物醫學研究(用來提升及支持衛生研究和醫學領域知識體系的基礎、應用或轉譯研究)的所有元素,都應將生理性別和社會性別納入考量。這個建議適用於各式的研究設計(調查、實驗、臨床試驗、田野調查、前瞻性研究、個案研究/對照實驗,及其它)並橫跨多種設計層面(見:健康與醫療檢核單)。考量研究設計時的重要資源包括:Oertelt-Prigione, S.等人,臨床醫學的生理性別和社會性別層面;Schenck-Gustafsson等人,臨床社會性別醫學手冊;以及Regitz-Zagrosek,藥理學中生理性別和社會性別之差異。其他的資源:美國國家衛生研究院線上課程「人類衛生之生理性別和社會性別科學」;線上課程「歐洲社會性別醫學課程」。
衛生研究關切各種影響個人或整體人口健康的因子,例如:保護和危險因子、衛生的社會決定因素、環境和資源分配(見:分析社會性別)。而用來評估藥物、儀器、診斷步驟和治療法之安全性和有效性的臨床研究,則能預防、治療、診斷或紓緩疾病的症狀,因此一般需要認真考量研究對象(選擇和納入條件,例如:抽樣)—見:分析生理性別。
抽樣(選擇或納入條件以及徵求策略) 
要尋求代表性樣本,且此研究結果要得以推及此取樣的群體時:甲)在研究開始前,決定可能影響結果的特徵;和乙)使用確保能徵求到足夠數量的受試者之徵求策略,不論對象是動物或是人類。目標是有足夠的統計檢力,以偵測結果的不同。有潛在臨床意義的變數包括:
- 生理性別:許多疾病的流行病學和許多診斷與治療的安全性和有效性會因生理性別而有所不同(見分析生理性別)。即使是多數標準且已經核可的介入措施,我們仍不應假設會從女性與男性身上觀察到相似的效果。當研究資料來自於單一生理性別(通常是只有男性),但是卻將結果推廣至兩性時,就可能產生實質性的傷害 (Kim et al., 2010)。女性生理性別對某些藥物有不良反應是一個危險因子,例如「芭蕾舞式心律不整」(torsades de pointes),這是一種威脅生命的心律不整(Gupta et al., 2007)。自1994年起,美國立法要求第三期以及其後的臨床試驗必須包含女性和少數民族/種族(1993年復興法案)。然而,
- 即使是美國國家衛生研究院復興法案管轄的臨床試驗,以在病人群中的代表性而言,女性仍相對招收不足。舉例來說,見下方心血管疾病的試驗。
- 復興法案並不適用於早期階段的醫學研究。美國國家衛生研究院特別指出「美國國家衛生研究院定義的『臨床試驗』是一個具有廣泛基礎的前瞻性第三期臨床調查,通常涉及數百個或更多的人類受測者,目的是比較實驗性的介入措施與標準或對照措施,或比較兩個或多個現有的治療,以進行評估」(美國國家衛生研究院,2001)。根據這個定義,一期或二期的研究不屬於臨床試驗,因此不受該法案的規定管制。當第一與第二期研究不包含女性時,可能因此錯失與生理性別相關的重要發現。舉例來說,可能因此無法開發只作用於女性的藥物。
- 將女性排除於預防出生缺陷或是其他對胎兒的傷害之臨床試驗之外是沒有道理的。在1950年代,開立利沙利度胺處方給孕婦造成大量的新生兒異常或死胎,從而「刺激了保護主義研究政策,諷刺的是,這項政策卻往往傷害婦女」(Gorenberg et al., 1991)。目前的政策對臨床試驗招收孕婦有嚴格的規範;招收條件要求「唯有當措施或程序是抱持對女性或胎兒有直接受惠之期望下進行時,才得以使胎兒暴露風險;或者,如果沒有這樣有利的前景,胎兒冒的風險不能大於最小值,且該研究的目的是為了無法藉由其他任何方式取得之重要生物醫學知識發展」(Department of Health and Human Services, 2001)。同樣嚴格的準則適用於醫學研究的兒童招募 (Code of Federal Regulations, 2009; European Parliament, 2001)。
在某些情況下,研究人員也將最嚴格的招收準則(設計用來保護孕婦)應用於具生殖潛力的女性(也就是所有介於初經和停經的女性),此舉有效地將這些女性排除在基礎研究外(Kinney et al., 1981)。這樣的「保護主義」政策,事實上可能危及孕婦,因為「新藥和器材通常未獲核准使用」於懷孕期間。(Baylis, 2010)。即使許多臨床試驗招收的病人群年齡中,多數或所有的女性都已停經,女性在這類研究的代表性仍顯不足。
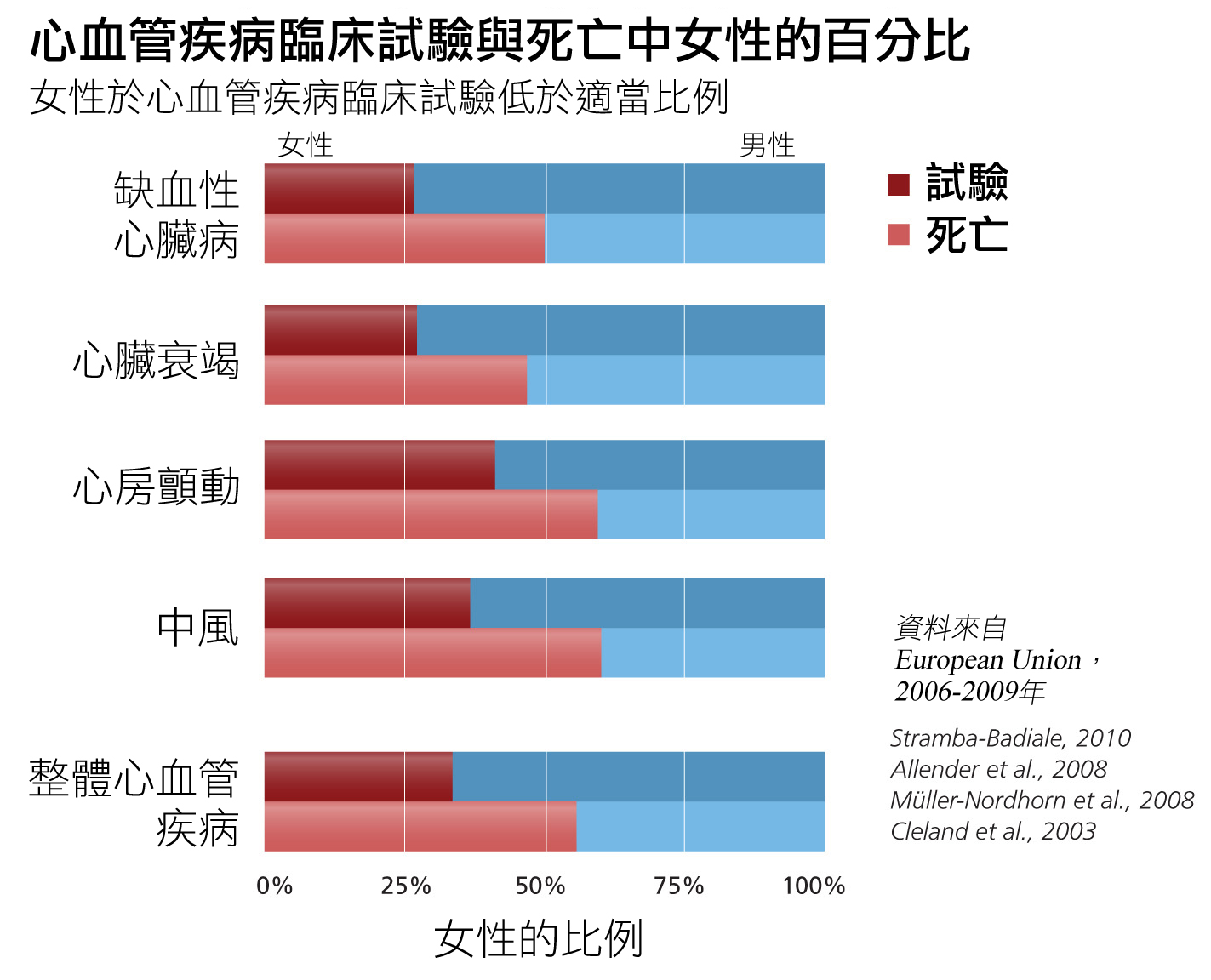
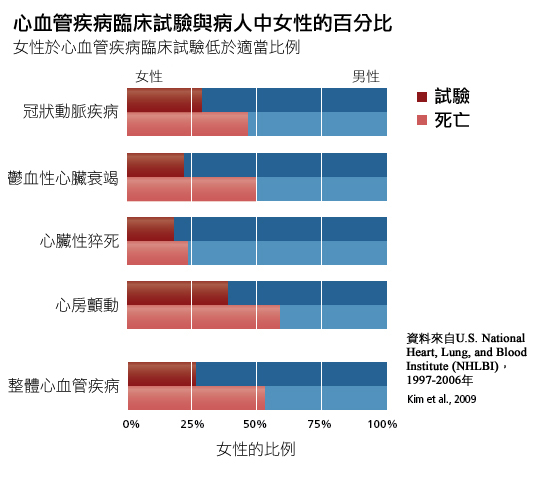
認知到此缺失後,歐洲藥品管理局(EMEA)主張,讓研究之族群的人口統計學資料能符合具有接受特定療程之資格的患者的人口統計學資料,是「藥物開發的基本原則」(EMEA, 2005)。以心血管疾病的具體情況而言,歐洲藥品管理局強調有必要針對「血脂、激素水平以及停經或身體組成的影響等」潛在生理性別差異進行研究,這可能有助於瞭解,為何從預後和有效的診斷檢驗會觀察到生理性別差異(EMEA, 2006)。
- 生殖(或激素)水平:雌性和雄性皆有顯著的激素變化:青春期時、老化期間、雌性之動情或月經週期、懷孕期間和期後,以及更年期轉換期間。這些激素變化具有廣泛的生理效應,造成免疫功能、體液平衡、體溫控制和身體組成等變化。研究人員應該適時在規畫的研究中考慮這些效果 (Becker et al., 2005)。
- 於月經週期的不同時期,抽樣自然排卵的女性(或是在雌性動物動情週期的不同時期)—見案例研究:動物研究。
- 將口服避孕藥、停經激素以及雄激素等外源激素的廣泛使用(和效果)納入考量。
- 在女性不同懷孕時期和產後進行抽樣。
- 於中年女性研究中,收集更年期之早期、晚期及其後狀況的資料。
- 社會性別行為:社會性別角色和身分會影響疾病的危險因子、治療和結果,因此,生物醫學研究可能需要將它們納入考慮。生物醫學重要的社會性別化因子可能包括:
- 與生理性別和社會性別交織的因子:年齡、身體組成以及共病率等變數通常可能和生理性別相關(或是一同變異),如果沒有將這些因子納入考慮,可能會混淆結果。舉例來說,由於心血管(CV)事件在男性身上診斷到的年齡比女性低,而許多心血管試驗又有年齡臨界值,因而多數心血管試驗中,符合資格的女性比例較低。此外,參加心血管試驗的女性,例如,與降低膽固醇藥物相關的實驗,其年齡普遍較高而且相較於男性,有較多(與年齡相關)的共病(Dey et al., 2009)。僅比較只有男性或是只有女性之心血管試驗的結果而不考慮年齡因素,可能導致對試驗發現的錯誤詮釋。
雖然研究人員同時從兩種生理性別採取足夠的樣本數是很重要的,但是在下列情況下,單一生理性別的研究可能比混合生理性別的研究好:
- 研究只影響女性或男性的狀況。這些通常是生殖器官的疾病,包括生理性別特有之癌症,例如:女性的卵巢癌以及男性的前列腺癌。其他單一生理性別疾病包括月經和停經狀況、懷孕和生產、以及勃起障礙。
- 已於單一生理性別進行廣泛測試的醫療診斷和介入措施試。舉例來說,只研究年輕男性之人類乳突病毒疫苗接種的安全性和有效性是合理的(Giuliano et al., 2011),因為過去已廣泛研究此疫苗於年輕女性的效果(Future II Study Group, 2007)。同樣地,許多骨質疏鬆藥物作用在女性的效果已受到廣泛地研究,但是尚未研究其作用於男性的效果。
- 已充分理解疾病的發展、診斷或治療差異(或相似—亦即之前的研究顯示無顯著差異)。在這種情況下,通常透過分析與生理性別相交的因子,單一生理性別研究可以讓研究人員檢查每個生理性別內的差異。舉例來說,女性之缺血性中風與評估(WISE)研究是評估沒有明顯阻塞性冠狀動脈疾病、但有心絞痛症狀之女性。他們注意到這種疾病在男性少見許多,也確定了缺血的數個根本原因 (Shaw et al., 2008)。
研究設計應建立於能確保研究招收之女性與男性會接受相同介入措施、以及保持相同水平的策略上,而且收集的資料是可以比較的。
在某些狀況下,可進行回顧性分析。舉例來說,研究人員可能於一組只有女性的研究中比較特定藥物的治療效果,並在一組只有男性的研究中比較同種藥物的治療效果。雖然兩組皆無法偵測生理性別差異,但兩組研究的整合分析可能可以顯示這類差異。然而相較於前瞻性分析,回顧性分析較不可靠—見常見的抽樣錯誤。
相關案例研究
參考資料
Allender, A., Scarborough, P., Peto, V., Rayner, M., Leal, J., Luengo-Fernandez, R., & Gray, A. (2008). European Cardiovascular Disease Statistics, 2008 Edition. Brussels: European Heart Network.
Baylis, F. (2010). Pregnant Women Deserve Better. Nature, 465 (7299), 689-690.
Becker, J., Arnold, A., Taylor, J., Young, E., Berkley, K., Blaustein, J., Eckel, L., Hampson, E., Herman, J., Marts, S., Sadee, W., & Steiner, M. (2005). Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology, 146 (4), 1650-1673.
Cleland, J., Swedberg, K., Follath, F., Komajda, M., Cohen-Solal, A., Aguilar, J., Dietz, R., Gavazzi, A., Hobbs, R., Korewicki, J., Madeira, H., Moiseyev, V., Preda, I., van Gilst, W., Widimsky, J., Freemantle, N., Eastaugh, J., & Mason, J. (2003). The EuroHeart Failure Survey Programme—A Survey on the Quality of Care among Patients with Heart Failure in Europe, Part 1: Patient Characteristics and Diagnosis. European Heart Journal, 24 (5), 442-463.
Code of Federal Regulations (CFR). (2009). Title 45 (Public Welfare), Part 46 (Protection of Human Subjects). Washington, D.C.: Government Publishing Office (GPO).
Department of Health and Human Services (DHHS). (2001). Protection of Human Research Subjects, Code of Federal Regulations (CFR) Title 45, Part 46, Final Rule. United States Federal Register, 66 (219), 56775-56780.
Dey, S., Flather, M., Devlin, G., Brieger, D., Gurfinkel, E., Steg, P., FitzGerals, G., Jackson, E., & Eagle, K. (2009). Sex-Related Differences in the Presentation, Treatment, and Outcomes among Patients with Acute Coronary Syndromes: The Global Registry of Acute Coronary Events. Heart, 95, 20-26.
European Medicines Agency (EMEA). (2006).Committee for Medicinal Products for Human USE (CHMP) Reflection Paper on Gender Differences in Cardiovascular Diseases. London: EMEA.
European Medicines Agency (EMEA). (2005). Gender Considerations in the Conduct of Clinical Trials. London: EMEA.
European Parliament and Council of the European Union. (2001). Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations, and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Official Journal of the European Communities, L121, 34-44.
Future II Study Group. (2007). Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. New England Journal of Medicine, 356 (19), 1915-1927.
Giuliano, A., Palefsky, J., Goldstone, S., Moreira, E., Penny, M., Aranda, C., Vardas, E., Moi, H., Jessen, H., Hillman, R., Chang, Y., Ferris, D., Rouleau, D., Bryan, J., Marshall, D., Vuocolo, S., Barr, E., Radley, D., Haupt, R., & Guris, D. (2011). Efficacy of a Quadrivalent Human Papilloma Virus (HPV) Vaccine against HPV Infection and Disease in Males. New England Journal of Medicine, 364 (5), 401-411.
Gorenberg, H., & White, A. (1991). Off the Pedestal and Into the Arena: Toward Including Women in Experimental Protocols. New York University Review of Law and Social Change, 19, 205-246.
Gupta, A., Lawrence, A., Krishnan, K., Kavinsky, C., & Trohman, R. (2007). Current Concepts in the Mechanishs and Management of Drug-Induced QT Prolongation and Torsade de Pointes. American Heart Journal, 153 (6), 891-899.
Institute of Medicine (IOM). (2010). Women’s Health Research: Progress, Pitfalls, and Promise. Washington, D.C.: National Academies Press.
Johnson, B., Shaw, L., Pepine, C., Reis, S., Kelsey, S., Sopko, G., Rogers, W., Mankad, S., Sharaf, B., Bittner, V., & Merz, C. (2006). Persistent Chest Pain Predicts Cardiovascular Events in Women without Obstructive Coronary Artery Disease: Results from the National Institutes of Health (NIH)-National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischaemia Syndrome Evaluation (WISE) Study. European Heart Journal, 27 (12), 1408-1415.
Kim, A., Tingen, C., & Woodruff, T. (2010). Sex Bias in Trials and Treatment Must End. Nature, 465 (7299), 688-689.
Kim, E., Carrigan, T., & Menon, V. (2008). Enrollment of Women in National Heart, Lung, and Blood Institute (NHLBI)-Funded Cardiovascular Randomized Controlled Trials Fails to Meet Current Federal Mandates for Inclusion. Journal of the American College of Cardiology, 52, 672-673.
Kinney, E., Trautmann, J., Gold, J., Vesell, E., & Zelis, R. (1981). Underrepresentation of Women in New Drug Trials: Ramifications and Remedies. Medicine and Public Issues, 95 (4), 495-499.
Müller-Nordhorn, J., Binting, S., Roll, S., & Willich, S. (2008). An Update on Regional Variation in Cardiovascular Mortality within Europe. European Heart Journal, 29 (10), 1316-1326.
NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research – Amended, October, 2001.
Oertelt-Prigione, S.& Regitz-Zagrosek, V. (Eds.) (2012). Sex and Gender Aspects in Clinical Medicine. London: Springer Verlag.
Regitz-Zagrosek, V. (Ed.) (2012). Sex and Gender Differences in Pharmacology. London: Springer Verlag.
Ridker, P., Cook, N., Lee, M., Gordon, D., Gaziano, J., Manson, C., Hennekens, H., & Buring, J. (2005). A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. New England Journal of Medicine, 352 (13), 1293-1304.
Schenck-Gustafsson, K., DeCola, P., Pfaff, D., Pisetsky, D. (Eds.) (2012). Handbook of Clinical Gender Medicine. Basel: Karger, 2012.
Stramba-Badiale, M. (2010). Women and Research on Cardiovascular Diseases in Europe: A Report from The European Heart Health Strategy (EuroHeart) Project. European Heart Journal, 31 (14), 1677-1685.
